July 7, 2016
New research in mice from the Weizmann Institute of Science may help explain how viral infection in pregnancy raises the risk of autism and schizophrenia in the child.
Recently published in Science and reported in the Jewish Post and elsewhere, disrupted foetal immune system development, such as that caused by viral infection in the mother, may be a key factor in the later appearance of certain neurodevelopmental disorders for the child.
The study may explain, among other things, how the mother’s infection with the cytomegalovirus (CMV) during pregnancy, which affects her own and her foetus’ immune system, increases the risk of her offspring developing autism or schizophrenia, sometimes years later.
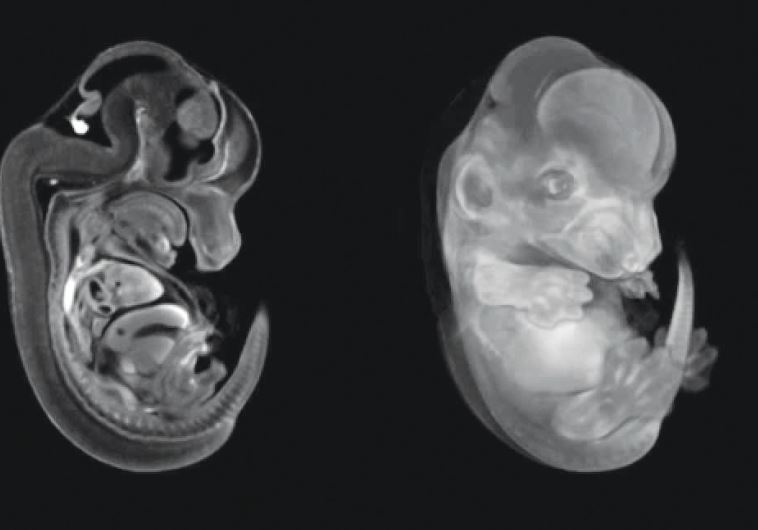
This increased risk of neurodevelopmental diseases was discovered many years ago in epidemiological studies and confirmed in mouse models with the Weizmann study, led by Dr Ido Amit and Professor Michal Schwartz, of the Immunology and Neurobiology Departments providing a possible explanation for this increase on the cellular and the mechanistic molecular levels.
“Previous studies showed that the timing of the disruption in the mother’s immune system during pregnancy affects the type of brain damage her child may develop. For example, a viral infection in early pregnancy raises the risk of autism, whereas an infection later in the pregnancy raises the risk of schizophrenia,” said Amit.
“We’ve set out to examine the mechanisms behind these phenomena, while focusing on the role the immune system plays in brain development.”
Orit Matcovitch-Natan, a graduate student in the laboratories of both Amit and Schwartz, and other members of the two teams, studied the sole immune cells present in the brain – the microglia, which contribute to the brain’s development and maintenance.
The scientists discovered that the development of these cells in the mouse foetus and in newborn mice proceeds in three distinct stages, parallel to those of the developing brain: early cells that populate the brain of the embryo shortly after its inception, pre-microglia and adult cells.
By screening the genomes of these cells and testing them extensively, the scientists were able to define each stage in terms of its activated genes, their control mechanisms and the epigenetic features, that is, the activation of proteins that “package” the DNA and affect gene expression in the course of development. The scientists also characterized the functions of some of these genes in the microglia, which contributed to an in-depth understanding of the developmental processes.
The second stage – that of the pre-microglia – proved the most sensitive to disruptions. This stage takes place close to birth and shortly afterwards, just when the developing brain undergoes the vital process of “pruning,” in which inappropriate synapses among neurons are lopped off. The pre-microglia play an important role in pruning, helping remove the superfluous neuronal networks, and shaping and strengthening the connections among the remaining neurons.
When the scientists exposed the brains of pregnant mice to synthetic materials that mimic a CMV infection, they found that the development of the pre-microglia was disrupted in their offspring. Genes involved in the maturation of these cells were expressed at the wrong time, and the cells proceeded to an adult stage earlier than usual. The offspring later exhibited abnormal behaviour, including disturbances in social interaction and behaviours similar to those of people with schizophrenia.
“We’ve discovered that it’s essential for the development of immune cells in the brain to be synchronized with the development of the brain itself, premature shift of the microglia in mice to the adult stage leads to brain malfunction later on,” said Schwartz.
Though these findings have been obtained in mice, the scientists hypothesize that disrupted coordination between the development of the microglia and that of the brain contributes to an increased risk of such neurodevelopmental disorders as autism and schizophrenia in human beings. The scientists believe that the heightened immune response to viral infection in the mother’s body may be responsible for disrupting the timing of microglia development.
“Our research has paved the way for studying the effects of other viruses on the mother’s immune system in general, and on her offspring’s brain development. It can also advance the study of neurodevelopmental disorders and their connection to the immune system,” added Orit Matcovitch-Natan.
In yet another series of experiments, the Weizmann scientists established a connection between the development of the microglia in the brains of mice and intestinal microbes – the microbiome. They found that in newborn mice that were free of any microbes, the maturation of the microglia was delayed. This finding suggests that in human babies, factors that shape the microbiome – natural ones such as breastfeeding, or therapeutic, such as antibiotics – may affect the immune cells in the baby’s brain and consequently the brain’s development.
It is still unknown to what extent this research in mice is relevant to human beings, but the scientists hope that an improved understanding of this process may in the future help prevent certain neurological disorders in babies, caused by disruptions in the mother’s immune system.
Taking part in the study were Dr Deborah R. Winter, Amir Giladi, Eyal David and Dr Hadas Keren-Shaul, as well as Dr. Eran Elinav and Christoph Thaiss of the Immunology Department, and Hila Ben-Yehuda, Merav Cohen and Dr Kuti Baruch of the Neurobiology Department. Matcovitch-Natan and Amit Spinrad, also a graduate student, belonged to both departments. Study participants also included Professor Michael H. Sieweke of Centre National de la Recherche Scientifique, France.