November 28, 2019
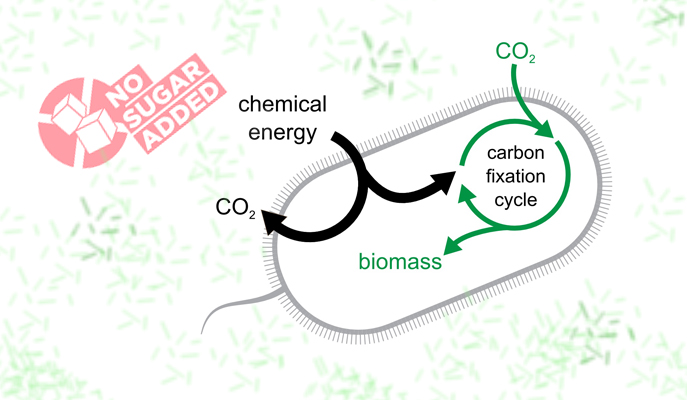
Bacteria in the lab of Professor Ron Milo at the Weizmann Institute of Science have not just sworn off sugar, they have stopped eating their normal solid food, existing instead on carbon dioxide (CO2) from their environment. This bacteria could contribute to new and future carbon-efficient technologies.
Reported recently in Cell, the bacteria were able to build all of their biomass from air. This feat, involved nearly a decade of rational design, genetic engineering and a sped-up version of evolution in the laboratory. The findings could help develop carbon-neutral fuels.
The study began by identifying crucial genes for the process of carbon fixation – the way plants take carbon from CO2 for the purpose of turning it into biological molecules such as protein or DNA. The research team then added and rewired the needed genes. They found that many of the ‘parts’ for the machinery that were already present in the bacterial genome could be used as is.
The team then inserted a gene that allowed the bacteria to get energy from a readily available substance called formate that can be produced directly from electricity and air and which is apt to ‘give up’ electrons to the bacteria.
However, just giving the bacteria the means of production was not enough to make the switch. There was still a need for another trick to get the bacteria to use this machinery properly, and this involved a delicate balancing act.
Together with Roee Ben-Nissan, Yinon Bar-On and other members of Milo’s team in the Institute’s Plant and Environmental Sciences Department, Dr Schmuel Gleizer used ‘lab evolution’ as the technique which gradually weaned the bacteria off the sugar they were used to eating.
At each stage, cultured bacteria were given just enough sugar to keep them from complete starvation, as well as plenty of CO2 and formate. As some ‘learned’ to develop a taste for CO2 – giving them an evolutionary edge over those that stuck to sugar – their descendants were given less and less sugar, until after about a year of adapting to the new diet some of them eventually made the complete switch, living and multiplying in an environment that served up pure CO2.
To check whether the bacteria were not somehow ‘snacking’ on other nutrients, some of the evolved E. coli were fed CO2 containing a heavy isotope – C13. Then the bacterial body parts were weighed, and the weight they had gained checked against the mass that would be added from eating the heavier version of carbon. The analysis showed the carbon atoms in the body of the bacteria were all extracted directly from CO2 alone.
The research team then set out to characterise the newly-evolved bacteria. What changes were essential to adapting to this new diet?
While some of the genetic changes they identified may have been tied to surviving hunger, others appeared to regulate the synchronization of the steps of making building blocks through accumulation from CO2.
“The cell needs to balance between toxic congestion and bankruptcy,” said Bar-On.
Yet other changes the team noted had to do with transcription – regulating how existing genes are turned on and off.
“Further research will hopefully uncover exactly how these genes have adjusted their activities,” qualified Ben-Nissan.
The researchers believe that the bacteria’s new ‘health kick’ could ultimately be healthy for the planet.
Milo points out that today, biotech companies use cell cultures to produce commodity chemicals.
“Such cells – yeast or bacteria – could be induced to live on a diet of CO2 and renewable electricity, and thus be weaned from the large amounts of corn syrup they live on today,” he said.
Bacteria could also be further adapted so that rather than taking their energy from a substance such as formate, they might be able to get it straight up — say electrons from a solar collector – and then store that energy for later use as fuel in the form of carbon fixed in their cells. Such fuel would be carbon-neutral if the source of its carbon was atmospheric CO2.
“Our lab was the first to pursue the idea of changing the diet of a normal heterotroph – or one that eats organic substances – to convert it to autotrophism, which means ‘living on air’,” said Milo.
“It sounded impossible at first, but it has taught us numerous lessons along the way, and in the end we showed it indeed can be done. Our findings are a significant milestone toward our goal of efficient, green scientific applications,” Milo concluded.
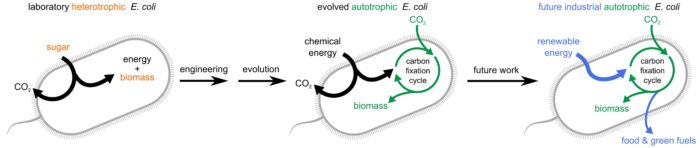
Researchers converted the common lab, sugar-eating (heterotrophic) E. coli bacterium (left) to producing all of its biomass from CO2 (autotrophic), using metabolic engineering combined with lab evolution. The new bacterium (center) uses the compound formate as a form of chemical energy to drive CO2 fixation by a synthetic metabolic pathway. The bacterium may provide the infrastructure for the future renewable production of food and green fuels (right)