
March 7, 2017
At times we all lose our nerve. But certain nerve losses, crucial during early nervous system development, may later play a role in diseases like amyotrophic lateral sclerosis (ALS) or peripheral neuropathy. New research at the Weizmann Institute of Science is uncovering the connection.
Like trees, neurons – the cells of our nervous system – sprout long thin branches called nerve fibers, or axons, which extend into all parts of the growing body.
During normal development, the immature nervous system grows an overabundance of these axons, the majority of which are deliberately ‘pruned’ back by the body. This ensures that only functional connections remain in the healthy nervous system.
However, once the nervous system has matured, any loss of axons and/or neurons is usually a response to acute injury or such diseases such as ALS or peripheral neuropathy.
These neurodegenerative diseases present differently in each patient, so scientists still do not understand the exact mechanisms and events that lead to the neurodegeneration that renders these diseases incurable and even lethal.
On the other hand, developmental processes are stereotypic and are therefore easier to study.
In research recently published in Neuron, Professor Avraham Yaron of the Weizmann Institute of Science’s Biomolecular Sciences Department and former PhD student Maya Maor-Nof and their team, discovered a new ‘gas and brake’ mechanism that controls the pace of axonal loss in developmental axonal pruning and showed that the genes involved are the same as those found in models of nerve injury.
To start with, the scientists mimicked the developmental process of pruning by first growing sensory neurons in a medium containing trophic factors – proteins that support axonal growth and the survival of specific groups of neurons – and then removing these trophic factors.
They then made a list of all the genes whose expression was elevated in response to the removal of trophic support. In a comparison with other sets of genes expressed in response to nerve injury, one gene in particular – Dusp16 – stood out, so they investigated this gene further to see if it plays a role in the degeneration process.
Surprisingly, they found that neurons that lacked Dusp16 – and therefore, its corresponding protein Dusp16 – which resulted in nerves and axons degenerating more quickly. This suggests that Dusp16 is an inhibitor or ‘brake’ that stops the nerve and axon degeneration process, and is not, as the scientists had expected, a promoter of degeneration.
To understand exactly how Dusp16 works, they returned to the list of expressed genes and found that an additional gene – Puma – was also shared between the developmental and injury paradigms. It was previously established that the Puma protein is part of a tightly regulated cell suicide program.
Could this somehow be connected to Dusp16 activity? Again, the scientists looked at neurons lacking Dusp16 and found that Puma increases in the absence of Dusp16, providing further evidence that Dusp16 has a protective function.
Upon further investigation, they revealed that an additional component – p53, a critical inducer of Puma – becomes ‘hyperactive’ in the neurons that lack Dusp16. The scientists were then able to link together the chain of events that occur under normal conditions: In response to trophic withdrawal, Dusp16 inhibits p53, which subsequently reduces the expression of Puma, thereby suppressing axonal degeneration.
“It came as a surprise that, rather than continuing on ‘full gas’ upon initiation of the pruning process, the neuron elicits an active program – in the form of Dusp16 – to prevent or slow down further axonal degeneration,” said Yaron.
“Our findings suggest the possibility that neurodegenerative diseases may work by ‘hijacking’ certain components of the immature nervous system developmental process; for instance, perhaps it causes a loss of inhibitors like Dusp16, or leads to an overexpression of pro-degeneration genes, such as Puma, to accelerate neurodegeneration.”
In a recent review published in Current Biology, Yaron and Professor Oren Schuldiner of the Molecular Cell Biology Department point out that discovering similarities between axon degeneration in both the developmental and the disease states may help pave the way to developing therapeutics to cure, or at least slow down, the symptoms of several debilitating diseases.
Professor Avraham Yaron’s research is supported by the Foundation Adelis; and the Maurice and Vivienne Wohl Biology Endowment. Professor Yaron is the incumbent of the Jack and Simon Djanogly Professorial Chair of Biochemistry.
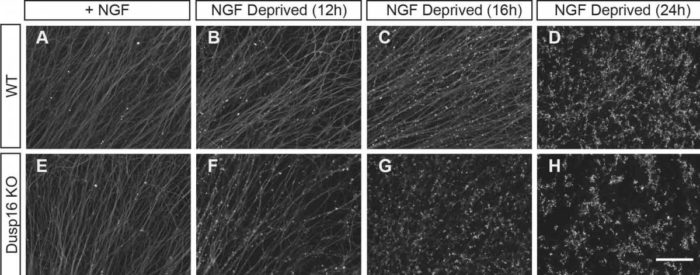
Upon trophic (NGF) withdrawal, axons degenerated earlier and at a faster rate in neurons lacking Dusp16 (bottom row) than the wild type neurons (top row), as can be seen from the punctuated pattern of axonal staining

(l-r) Valeria Ulisse, Hadas Sar Shalom, Professor Avraham Yaron, Dr Erez Romi and Dr Dena Leshkowitz






