
January 23, 2024
A new kind of immunotherapy, based on crosstalk between different immune cells, could pave the way for innovative treatments of cancer and autoimmune diseases
Winning on the battlefield takes a combination of precise intelligence and resolute soldiers. But when it comes to the battle against cancer, the immune system’s fighters – the T cells – quickly lose their ability to kill and become exhausted, while the intelligence-providing dendritic cells are scarce. This is one of the reasons that the great promise of immunotherapy – a new generation of treatments that harness the body’s own immune system for fighting cancer – has not been fully realised.
In a study published in Cell, Weizmann Institute of Science researchers present a newly developed antibody that connects T cells to dendritic cells, creating a powerful immune response to cancerous growths. The research opens a new avenue in immunotherapy: development of treatments that link up various cells in the immune system to create a crack combat team for defeating cancer and other diseases.
One of the most prominent immunotherapies makes use of antibodies that block PD-1, a regulatory ‘checkpoint’ receptor found on the surface of T cells. When this receptor is expressed in T cells, a protein widespread in the tumour environment can attach to it, switching the T cells into a state known as exhaustion. PD-1 antibodies prevent this protein from attaching to T cells and repressing them, but many cancer patients fail to respond to this treatment; in many others, the effectiveness is short-lived.
To develop a more effective immunotherapy, researchers from the laboratories of Dr Rony Dahan and Professor Ido Amit in Weizmann’s Systems Immunology Department started by asking why existing treatments fall short. To answer this question, they sampled T cells from two mouse models of cancer that had undergone PD-1 antibody treatment.
“Using advanced technologies such as single-cell DNA sequencing and big data algorithms, we examined almost 130,000 T cells, some of which responded to the treatment and some of which did not,” Amit explained.
“Surprisingly, the group of T cells that did respond to the treatment expressed genes pointing toward an interaction with a rare population of dendritic cells.”
Dendritic cells collect intelligence from across the body by swallowing molecules belonging to malignant cells. They then present their findings to T cells, thereby warning them about the cancerous growth and prompting them to take action. PD-1 antibodies are supposed to help with activating the T cells that fight cancerous growths, but when the researchers examined a cancer model from a mouse lacking dendritic cells, they discovered that the antibody treatment had lost its effectiveness entirely.
In other words, they revealed that dendritic cells are vital to the multiplication and activation of specific T cells in the fight against cancer and are therefore necessary for the treatment as a whole to be successful.
These findings exposed a key weakness in existing treatments: the fact that the relevant population of dendritic cells is rarely present in most cancerous growths and in most patients currently being treated with PD-1 antibodies. Under these conditions, interaction between these cells and the T cells that they activate rarely takes place.
This understanding paved the way for the engineering of a new antibody called BiCE (Bispecific DC-T Cell Engager), whose two arms were designed to connect two different types of cells: One arm binds to T cells, inhibiting the PD-1 receptor, just as existing treatments do; the other arm recruits the dendritic cells from the rare population that is vital for activating the T cells.
The development of the new treatment was led by doctoral research students Yuval Shapir Itai, from Dahan’s laboratory, and Oren Barboy, from Amit’s laboratory.
Having created their antibody, the researchers studied its mechanism of action. When they used fluorescent markers to label the antibody and the immune cells of mice with skin cancer who had been given the new treatment, they were able to observe how the antibody physically connects the T cells to the dendritic cells, increasing the number of such cellular pairs around the cancerous growth and in the adjacent lymph nodes.
They also discovered that the cellular pairs created by the antibody were active and that they triggered an immune response against the growth. Moreover, in the wake of the treatment, the dendritic cells that had been adjacent to the cancerous growth migrated to the lymph nodes and connected to the T cells there, in order to share information and activate them.
New hope for untreatable diseases
The effectiveness of this new treatment was tested in several mouse models of cancer, including aggressive breast, lung and skin cancers.
Treatment with the new antibody, as compared to the existing treatment, significantly reduced the growth rate of the skin and lung cancers. In contrast, breast tumours that did not respond to the existing treatment also failed to respond to the new antibody. Researchers believe that this is because of the very small number of active dendritic cells around these tumours.
Therefore, they tried combining their new antibody with an existing treatment that enhances the activity of dendritic cells around the growth. This combined treatment was found to be more effective than the existing options. It proved that even in cancers that had not responded to immunotherapy until now, the synergy between T cells and active dendritic cells creates a powerful immune response against the tumour.
The next stage of the study was to examine whether, alongside a powerful immune response against the primary cancerous growth, the new antibody also manages to prevent the disease from returning.
Many cancer patients suffer such a recurrence even after the primary growth has been removed and any known metastases have been treated. The main danger is the existence of tiny remnants of the disease that escape detection and start developing later, causing the tumour to recur. BiCE, unlike existing treatments, has been found to be effective in thwarting the development of metastases in the lungs after the removal of the primary tumour. This might be evidence that the antibody manages to create a systemic immune response against the cancer throughout the entire body, and that after treatment, it leaves behind immune cells that remember how to identify the cancer and respond accordingly.
Yeda Research and Development, which is responsible for commercializing the intellectual property of Weizmann Institute scientists, has filed a patent application and is working to develop an innovative treatment based on the Weizmann antibody.
“We are presenting a new approach that puts the emphasis on a system view of immunotherapy,” said Dahan.
“Instead of looking at one avenue, we engineer antibodies that serve as a communication platform between whichever immune cells we choose. This development gives hope not only to cancer patients, who need to have their immune systems activated to fight off the growth, but also to people with other diseases, such as autoimmune diseases, in which patients need a suppression of the immune response against their own body,” he said.
“There are ways of suppressing the entire immune system, but our new approach should make it possible to suppress or activate a targeted immune response, without the broad and dangerous ramifications of overall suppression and activation of the immune system.”
Also participating in the study were Drs Ran Salomon, Ken Xie and Eitan Winter of Weizmann’s Systems Immunology Department; Akhiad Bercovich and Professor Amos Tanay of Weizmann’s Computer Science and Applied Mathematics Department; Tamar Shami and Professor Neta Erez from Tel Aviv University’s Faculty of Medicine; and Dr Ziv Porat of Weizmann’s Life Sciences Core Facilities Department.

(l-r) Dr Rony Dahan, Yuval Shapir Itai, Oren Barboy and Professor Ido Amit
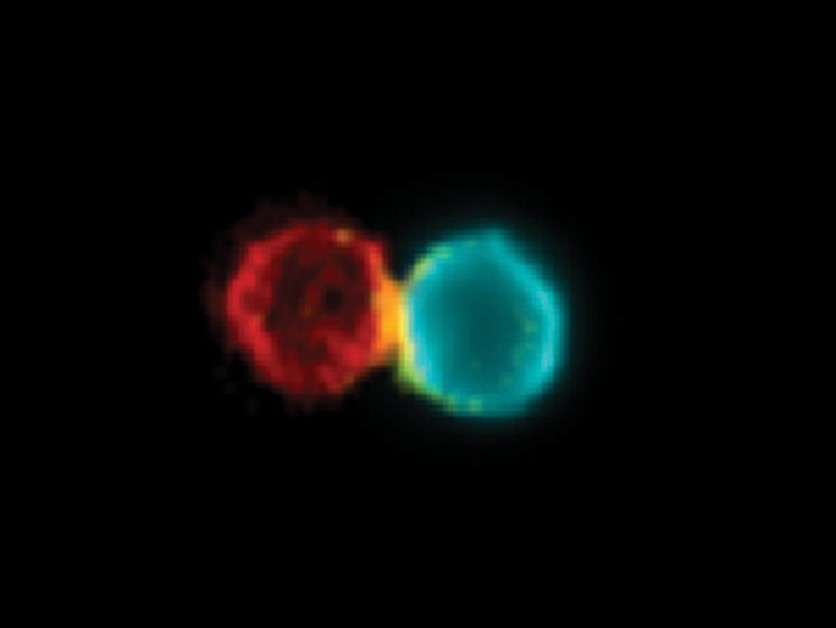
Combat team: A dendritic cell (right) and a T cell, both from a mouse with skin cancer, combined using the BiCE antibody (yellow)
DrawImpacts, Emma Vidal






