March 17, 2019
Antifreeze is life’s means of surviving in cold winters: Natural antifreeze proteins help fish, insects, plants and even bacteria live through low temperatures that should turn their liquid parts to deadly shards of ice. Strangely, in very cold conditions, the same proteins can also promote the growth of ice crystals, which was the finding of experiments carried out in Israel and Germany using proteins taken from fish and beetles.
The results of this study, recently published in The Journal of Physical Chemistry Letters, could have implications for understanding the basic processes of ice formation.
Antifreeze proteins do not prevent ice from forming in the first place. They wrap themselves around tiny ice crystals – the nuclei that provide the ‘template’ for growing larger ice crystals – and stop them from growing. Flour beetle larvae, for example, have such proteins on their outer shells, to keep away ice that could break their fragile skin.
The scientists wanted to compare the antifreeze proteins to natural proteins that can promote the growth of ice crystals. Some bacteria, for example, are known to grow sharp ice crystals that then split the skins of ripe tomatoes. Although it was believed that these two kinds of protein were very different, previous scientific studies suggested that they were more similar than thought.
The basic premise was based on the idea that antifreeze proteins have an active site that can bind to ice; and an ice binding site can support the formation of an initial ice nucleus that has the potential to grow into an ice crystal. The problem was that, until now, there had been little way to truly isolate the actions of these biological molecules.
The present study was led by Professor Thomas Koop of Bielefeld University in Germany and in collaboration with Professor Ido Braslavsky’s group at the Hebrew University of Jerusalem and Professor Yinon Rudich of the Weizmann Institute of Science.
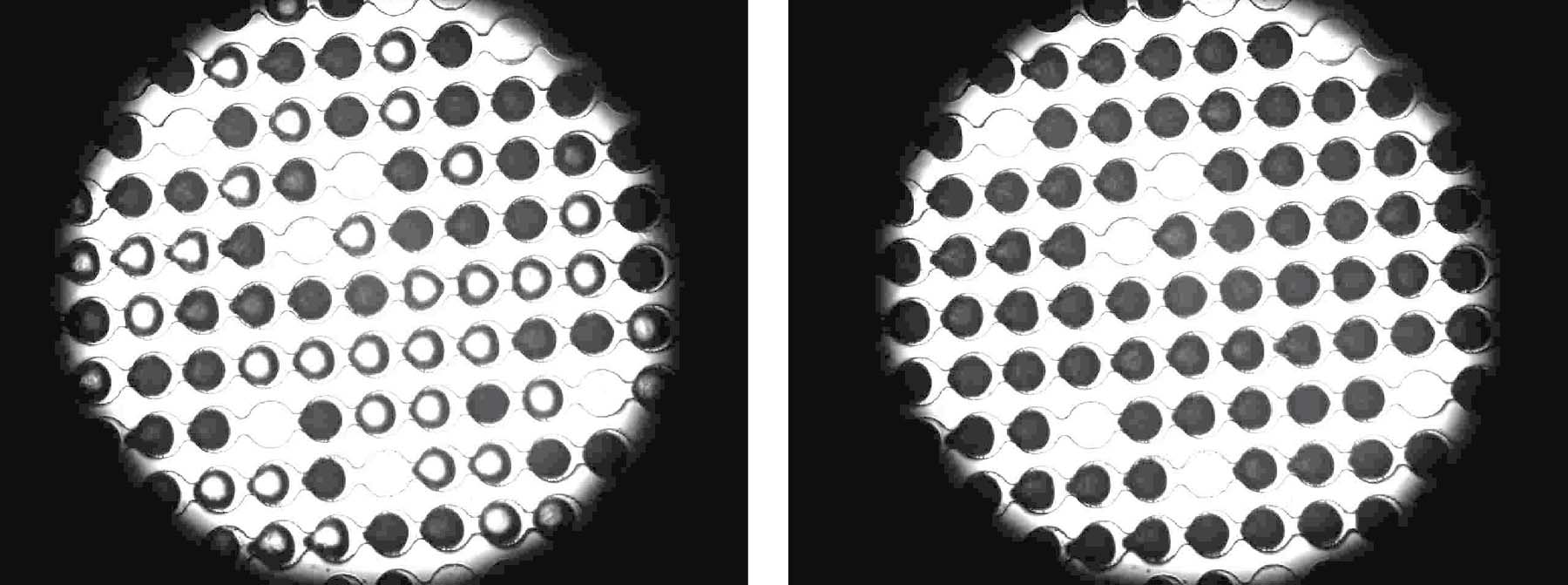
It was made possible by a device developed in Professor Yinon Rudich’s group, which they dubbed WISDOM (Weizmann Supercooled Droplets Observation on a Microarray). This microfluidic device has micron- sized channels and droplet traps that enabled the researchers to capture microdroplets of ultra-pure water on each chip. They then added carefully measured amounts of antifreeze proteins purified from flour beetle larvae or from a fish that lives in the arctic year-round.
Once the antifreeze proteins were added to the droplets, they were cooled to chilling temperatures. The water was still liquid, even though it had already been cooled to well below its normal freezing point (thus, supercooled), in part because it was lacking the impurities that normally make our water turn into ice cubes at 0 degrees.
Ice formed in the samples only as the water temperature dropped below minus 30. This setup enabled the group to be sure that any ice-forming or -preventing activity was solely due to the actions of the proteins.
While in pure water microdroplets with nothing added, ice would begin to form at about 38.5 degrees below zero, and in around half of the samples with antifreeze proteins, ice crystals began forming at a higher temperature – close to minus 34.Therefore at certain temperatures, which are extreme but not unknown on the planet, the antifreeze actually becomes pro-freeze, initiating the growth of ice crystals.
The group compared these findings to what is known about the natural proteins that promote the growth of ice crystals – ice-nucleating proteins (INPs). These can efficiently form ice at higher temperatures than those in which the antifreeze proteins switched to ice production. The scientists posit that the main difference is in the size of the proteins – INPs are substantially larger. The finding adds to our understanding of both ice formation and prevention.
For Professor Rudich, whose work focuses on atmosphere and climate, it may help shed light on the physical processes that affect cloud formation, in which proteins and other complex molecules have an impact on the development of ice crystals in clouds.
Antifreeze proteins like those in the fish are used today, among other things, to keep ice cream smooth and to keep outside surfaces frost-free. This study suggests that these proteins may have limitations, and could actually promote ice build-up when exposed to extremely cold temperatures such as those that hit the North American continent this year. INPs have their uses as well, for examples in ski resorts that want to extend their seasons, so this study on antifreeze proteins could even point to ways of creating better ice-forming ones.
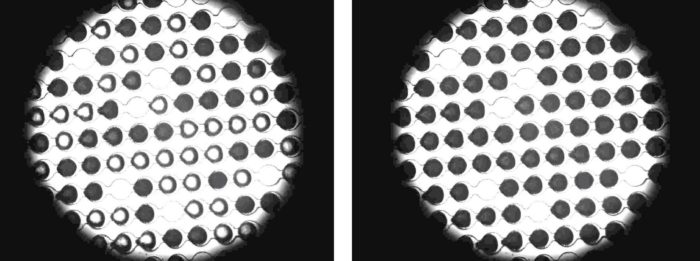
View of a research chip through a microscope: a high concentration of antifreeze proteins ensures that the drops freeze at temperatures that are less cold than usual (frozen drops are dark). Photo: Bielefeld University