April 24, 2021
If you dance cheek-to-cheek with a partner, your rhythms soon synchronise, so you move smoothly across the floor. Heart cells that beat – cardiomyocytes – are the same.
If you grow embryonic heart cells on a flexible material, you can not only observe this synchronisation, you can get these cells to change the rhythm of their beats from ‘rhumba’ to ‘waltz’ or vice versa, just by jiggling them so they ‘feel’ a new pace. But you’ll have to keep the jiggling up for tens of minutes to get them to make the transition, and once you stop, it will take the cells a similar amount of time to return to their normal pulse.
That finding arose from experiments done in the lab of Professpr Shelly Tzlil, of the Technion – Israel Institute of Technology. Professor Sam Safran, a theoretical physicist at the Weizmann Institute of Science’s Chemical and Biological Physics Department said this was puzzling. Together with his PhD student Ohad Cohen, Safran had previously shown that the spontaneous beating of cardiomyocytes – which occurs at a frequency of about once per second – could be described using an analogy to a mechanical system akin to a vibrating spring.
The puzzle was this: If, for the spring-like oscillations, a beating cycle takes place about every second, why is the time needed to get it to change its pace so much longer? Over an hour, the cell would contract 3,600 times – that is, if it were at a dance, a cell would keep waltzing while its partner jitterbugged until it was nearly time to leave.
If the only characteristic of the spontaneously beating cardiomyocyte were hat spring-like mechanism, the physics of such a system would dictate that it would react within the short timescale of the vibration, and new rhythms would be adopted within several seconds, not many minutes.
“When such a mismatch in timing happens, you first examine the intrinsic properties of the system, itself – even in the absence of forces that change its pace – and ask if a different characteristic timescale is involved,” said Safran.
Safran and Cohen suspected that a possible longer timescale might have to do with the intrinsic response of a spontaneously beating cell to the noise – the ‘fuzziness’ often seen in data from living systems – that was noted in the previous experiments. That is, cardiomyocytes beat about once a second – sometimes in nine-tenths of a second, sometimes waiting an extra tenth of a second to contract. Could an additional, slower mechanism be functioning in the cells to keep that noise close to the ‘ideal’ one second mark?
To test this idea, Tzlil grew cardiomyocytes in her lab and, working in collaboration with Cohen and Safran, timed the natural, unperturbed beating of individual cells for several hours.
The researchers thus obtained ‘cardiograms’ of these single cells. Viewing the data over the course of a minute or so, they plotted the difference between the actual timing of each contraction and its ‘ideal’ average timing, plus or minus, from the one-second mark.
More challenging was the plotting of beats over hours, rather than minutes, but these revealed a new pattern: The pulses that were slower or faster than average appeared in bunches – many slower beats, followed by many faster beats. The timescale associated with these bunches was tens of minutes, a far cry from the natural one-second timing of an ‘average’ beat.
It was as if a somewhat clumsy, invisible hand were continually adjusting the knob on a static-sounding radio station, turning the frequency up and down in an attempt to hit the exact right interval. This pattern of regulation occurred over a span of ten to thirty minutes.
When the team then compared the regulation from different lab-grown cardiomyocytes, after first mathematically accounting for the differences in their ranges (so that a regulation cycle taking, say, 15 minutes could be compared with one taking twenty-five), they found that the graphs looked very similar, implying that such a slow, noise-regulating mechanism is intrinsic to the functioning of all of these heart cells, no matter how fast or slow they beat.
How did the heart cell ‘know’ that it was beating too quickly or slowly, and why did it take it so long to compensate for faster or slower beating by introducing the opposite pace?
“Though we still don’t know the detailed biochemistry that accounts for this long regulation time,” said Safran.
“Its scale of tens of minutes suggests that gene transcription and protein translation are involved, possibly to modify the channels and pumps that area involved in the transport of calcium within the cell.”
Further experiments may reveal whether the duration of the regulation time is an indicator of cellular ‘good health’; too long a regulation time might mean that the cell is not oscillating properly over longer intervals. Extending the research to heart tissue may reveal the functional importance of overly long regulation times to the integrity of the beating tissue and perhaps in the future, to the heart itself.
“We showed, once again, that even as heart cells are noisy – in the manner of all biological systems – the noise is interesting; using physics to analyse its patterns reveals how the cell attempts to deal with its imperfect behaviour,” he added.
Beating cardiomyocytes in the lab of Professor Shelly Tzlil, the Technion
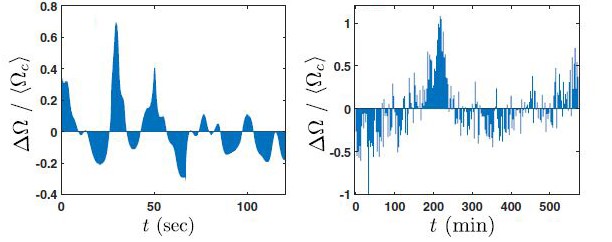
(l) Measured over a few minutes, cardiomyocyte beats regularly deviate up and down from the one second mark. (r) But seen over several hours, a longer oscillation around the one-second goal can be seen
Professor Sam Safran (sitting) and Ohad Cohen






