
September 10, 2021
A novel imaging method reveals a surprising arrangement of DNA in the cell’s nucleus as reported by the Weizmann Institute of Science.
If you open a biology textbook and run through the images depicting how DNA is organized in the cell’s nucleus, chances are you’ll start feeling hungry as the chains of DNA look like a bowl of ramen: long strings floating in liquid.
However, according to two new studies – one experimental and the other theoretical – that are the outcome of the collaboration between the groups of Professor Talila Volk of the Molecular Genetics Department and Professor Sam Safran of the Chemical and Biological Physics Department at the Weizmann Institute of Science, this image should be reconsidered. Clarifying it is essential since DNA’s spatial arrangement in the nucleus can affect the expression of genes contained within the DNA molecule, and hence the proteins found in the cell.
This story began when Volk was studying how mechanical forces influence cell nuclei in the muscle and found evidence that muscle contractions had an immediate effect on gene expression patterns.
“We couldn’t explore this further because existing methods relied on imaging of chemically preserved cells, so they failed to capture what happens in the cell nuclei of an actual working muscle,” she said.
To address this issue, Dr Dana Lorber, a research associate in Volk’s group, led the design of a device that makes it possible to study muscle nuclei in live fruit fly larvae. The device holds the tiny, translucent larva within a groove that allows it to contract and relax its muscles but keeps its movement constrained so that it can be scanned by a fluorescence microscope. Using the device, the researchers obtained images of the internal, linearly-organized complexes of DNA and its proteins (known as chromatin), surrounded by the membrane of the muscle nuclei.
Expecting a bowl full of ramen, Lorber and Dr Daria Amiad-Pavlov, a postdoctoral fellow in Volk’s group, were in for a surprise. Rather than filling up the entire volume of the nucleus, the ‘noodles’, or long chromatin molecules, were organized as a relatively thin layer, attached to its inner walls. Similar to the outcome of the interaction between oil and water, what is known as ‘phase separation’, the chromatin separated itself from the bulk of the liquid inside of the nucleus and found its place at its outskirts, while most of the fluid medium remained at the center. The researchers realized that they were on their way to addressing a fundamental biological question, that is – how is chromatin, and hence DNA, organized in the nucleus in a living organism.
“But the findings were so unexpected, we had to make sure no error had crept in and that this organization was universal,” Lorber said.
After teaming up with Safran’s group, they came to the conclusion there’d been no mistake. Safran and postdoctoral fellow Dr Gaurav Bajpai built a theoretical model that included the physical factors governing chromatin organization in the nucleus, such as the relative forces of attraction between chromatin and its liquid environment and between chromatin and the nuclear membrane.
The model predicted that the chromatin should undergo separation from the liquid phase, depending on the relative amount of liquid (hydration) in the nucleus. Furthermore, the phase separated chromatin could then arrange itself along the inside of the nuclear membrane – just as Volk’s team had found in their experiments.
The groups also explained why in previous studies by other scientists, the chromatin appeared to fill the cell nuclei.
“When scientists plate cells on a glass slide in order to study them under a microscope, they change their volume and physically flatten them. This may perturb some of the forces governing chromatin arrangement and reduce the distance between the upper part of the nucleus to its base,” Safran explained.
To make sure these findings were not limited to fruit fly muscle cells, Lorber and Amiad-Pavlov joined forces with Dr Francesco Roncato from Professor Ronen Alon’s group of the Immunology Department and examined live human white blood cells. In this case too, the chromatin was similarly organized as a layer lining the inner nuclear wall.
“This showed that what we’d found was likely to be a general phenomenon, and that this chromatin organization had probably been conserved throughout evolution,” said Amiad-Pavlov.
The study opens up new avenues of research into DNA’s organization in the cell and, by extension, into the physical forces that act upon the nucleus and chromatin that can affect gene expression.
One potential direction is exploring whether there’s a difference between DNA organization in health and disease. If so, this difference may be exploited in diagnosis, for example, as a new parameter for detecting cancer cells. In the study of embryonic development, exploring DNA organisation may help clarify whether mechanical forces affect the differentiation of cells into new fates.
Finally, it is known that stiffness of the surface on which cells are placed can alter the expression of their genes. The new study suggests this may have to do with the surface’s push and pull on the nuclear membrane and the resultant impact on DNA organization within the nucleus. A better understanding of this interplay may help control gene expression in cells employed for engineering tissues with desired properties.
Also taking part in the experimental study was Dr Adriana Reuveny from Professor Talila Volk’s group in the Molecular Genetics Department.
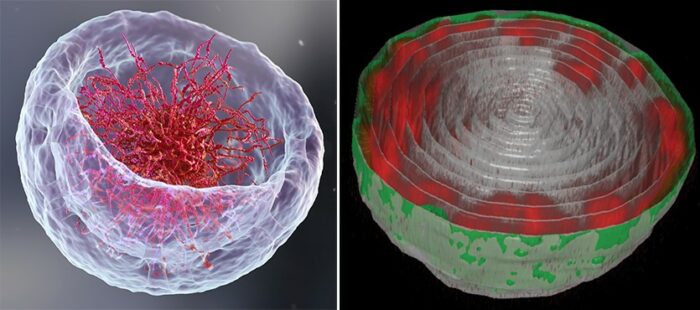
Left: A 3D illustration of the nucleus representing the classical theory of DNA organization at its center. Right: Turning the ramen bowl on its head - Microscopic image of the nucleus of a fruit fly larva’s muscle cell. The long chains of DNA (red) are attached to the nuclear lamina (green) – the inner layer of the nuclear membrane

(l-r) Professor Talila Volk, Professor Sam Safran, Dr Dana Lorber, Dr Daria Amiad-Pavlov and Dr Adriana Reuveny. Moving away from the center

Dr Gaurav Bajpai
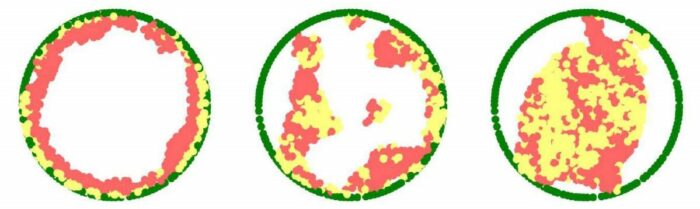
3D chromatin simulations reveal that chromatin organization in the nucleus is dependent on the physical interaction between chromatin and the nuclear lamina. When these interactions weaken (left to right) – as is the case in several diseases ranging from muscle dystrophies to neurological disorders – the chromatin shifts from the periphery of the nucleus to its center






