
August 31, 2024
Weizmann Insitute scientists have mapped common types of brain tumours at unprecedented resolution – and identified a possible reason why some patients fail to respond to a new drug.
The cells that make up cancerous brain tumours are extremely varied and sometimes create unique three-dimensional shapes. As far back as 1932, American neurosurgeon Percival Bailey attempted to label these cells and discovered that they can be divided into several families of cells with similar properties.
More than ninety years later, we still know precious little about the identities of cell groups that make up different kinds of brain tumourss, these groups’ organization and how they affect the course of the disease and the outcome of treatment. This is why the success rate for treatment of most brain cancers is typically not high.
Over the past decade, genetic sequencing technology that works at the single-cell level has made it possible to examine, in minute detail and in one fell swoop, thousands of cells in the same tissue, to understand which genes they express and to then categorise them and study the role of each group.
Scientists in Dr Itay Tirosh’s research group in the Weizmann Institute of Science’s Molecular Cell Biology Department, in collaboration with Professor Mario L. Suvà’s lab at Massachusetts General Hospital, harnessed this technology in order to reexamine some of the unanswered questions in the field of brain tumours.
The most common type of primary brain tumour is the glioma, which originates from the support cells that assist our nerve cells. There are two main types of glioma tumours: those that are usually less aggressive and have a mutation in the gene encoding an enzyme called IDH, and those without this mutation, which are highly aggressive and known in medical terminology as glioblastoma.
In the past few years, researchers from Tirosh’s lab have been using single-cell RNA sequencing to analyse the cellular composition of both kinds of tumours. They revealed that the tumour cells are divided into groups, each of which expresses a unique genetic program that determines the biological ‘state’ of the cancer cells in this group. Among other findings, the researchers discovered groups of cells that use their unique genetic programs to mimic normal brain cells.
In a new study published recently in Cell, researchers from Tirosh’s lab – led by Dr Alissa Greenwald, Noam Galili Darnell and Dr Rouven Hoefflin – harnessed technologies that make it possible to not only sequence the RNA on the single-cell level but also spatially map its expression.
For the first time, this allowed them to identify which genes are uniquely expressed in each of the thousands of areas within a brain tumour. As a result, they were able to precisely map how glioblastoma and glioma tumours are organized. To conduct the study, they took biopsies from 13 patients with glioblastomas and from six patients with gliomas that had the IDH mutation.
The researchers’ first discovery was that the groups of various cells within a glioma tumour are not distributed evenly across the tumour; rather, they are concentrated in various environments inside the growth. These microenvironments are not entirely homogenous: Cells from other groups were always found in proximity to other types of cells. In the next stage of the study, the researchers checked whether there were groups of tumour cells that usually exist in proximity to each other. They discovered that the cells not only had preferred neighbours but also that these good-neighbour couplings were consistent in different patients.
Certain neighbouring pairs imitated the natural behaviour of brain tissue. For example, cells that imitate the parent cells of the oligodendrocyte support cell were found close to endothelial cells, which line the walls of blood vessels. This coupling also occurs in healthy tissue, since endothelial cells release substances that are vital for the survival and proliferation of oligodendrocyte precursor cells. Similarly, cells that imitate neuron progenitor cells were found in the parts of the tumour that penetrated healthy brain tissue, just as progenitor cells in healthy tissue migrate when the tissue is regenerated.
Taking an overview to gain a fuller understanding of these couplings, the researchers realised that the cells created five distinct layers by organising themselves into separate environments within the tumour. The innermost layer – the core of the tumour – is made up of necrotic cells, which do not receive enough oxygen to survive. In the layer surrounding the necrotic core, the researchers found cells similar to embryonic connective tissue, as well as additional cells, including immune system cells responsible for causing inflammation. The third layer was primarily made up of blood vessels, endothelial cells forming blood vessel walls and additional immune system cells.
Cells in the two outer layers of the tumour don’t suffer from a lack of oxygen. This enables groups of tumour cells that mimic healthy brain tissue – progenitors of neurons and support cells – to develop in the fourth layer. The fifth, outermost layer contains healthy brain tissue, into which the tumour penetrates. These findings about the different layers of a tumour indicate that the driving force behind the tumour’s layered structure is the lack of oxygen, which is exacerbated as the disease progresses and the tumour develops.
Based on these findings, the researchers noticed a much more chaotic structure in less aggressive tumours – which are also usually smaller – and in areas of the tumour with a plentiful supply of oxygen. In most glioma tumours with the IDH mutation, for example, there was usually no necrotic tissue, and the structure of the tumour was disorganised; in the rare cases when there was necrotic tissue, the biopsies also showed a relatively well-ordered structure.
“We discovered that an organised spatial structure is characteristic of the more aggressive tumours,” Tirosh explains. “The lack of oxygen in the tumour cells’ environment influences the gene program that they express and therefore affects their state. As the tumour grows, distinct layers are formed, some of which may be less accessible to drugs and to cells from the immune system, and these could make the tumour more resilient.”
The changing status of cancer cells
Researchers from Tirosh’s lab used the information they collected on the cellular composition of glioma tumours to work out how a new, promising drug helped some of the patients with this type of cancer. To do so, they used biopsies from tumours of three patients who had participated in a clinical trial of the new drug and who had responded to the treatment, as well as biopsies from six patients who had not undergone any treatment. To complete the picture, they also used data from biopsies taken from an additional 23 patients who had taken the drug and 134 patients who had not.
The research team, led by Dr Avishay Spitzer, found that the drug, which works by inhibiting the mutant IDH enzyme, caused the cells to alter the gene program that they expressed. In fact the treatment encourages the cancerous stem cells to differentiate into mature cells, thereby undermining their ability to divide rapidly, blocking the disease’s progress.
The researchers postulated that if the drug works by causing cancerous cells to differentiate into mature cells, the mutation attacking the gene that is critical to the differentiation process could explain those cases in which the drug does not work. In the biopsies taken from patients who did not receive the drug, they identified a certain gene that is linked to low levels of mature cancerous cells. When they silenced that gene in a mouse model of cancer, they found, as expected, that the drug did not work. “This indicates that the gene mutation we identified could be a biological marker allowing us to determine in advance which patients will benefit from the treatment and which will not,” Tirosh explains. These new findings could also help find a course of treatment that combines IDH inhibitors with another drug that encourages the differentiation process and increases the treatment’s impact on the tumour.
“Our two most recent studies revealed the forces that shape the character of cancerous cells in a tumour, be that in their untouched environment or in one resulting from a therapy that alters the cells’ genetic program,” Tirosh says. “These findings pave the way for a new approach to cancer treatment, since once we are familiar with the cell groups that populate every area of the tumour and we know how a cell can move from one state to another, we might be able to develop new targeted treatments that will alter the course of the disease. The understanding that both the composition of cells within the tumour and its three-dimensional structure are linked to the level of the tumour’s aggressiveness could also lead to new diagnostic methods that do not rely solely on the volume of the tumour and the mutations it contains.”
Participants in the structural study of glioma also included Dr Dor Simkin, Yotam Harnik and Dr Julie Laffy from Weizmann’s Molecular Cell Biology Department; Dr Christopher W. Mount, Dr Nicolas Gonzalez Castro, Sydney Dumont, Dr Masashi Nomura and Vamsi Mangena from the Massachusetts General Hospital and Harvard Medical School in Boston, MA, USA; Dana Hirsch from Weizmann’s Veterinary Resources Department; Tom Talpir from Weizmann’s Computer Science and Applied Mathematics Department; Dr Merav Kedmi, Dr Inna Goliand, Dr Hadas Keren-Shaul and Dr Yoseph Addadi from Weizmann’s Life Sciences Core Facilities Department; Gioele Medici, Professor Michael Weller and Dr Marian C. Neidert from University Hospital Zurich, Switzerland; and Dr Baoguo Li from Weizmann’s Systems Immunology Department.
Participants in the IDH inhibitor study also included Dr Rony Chanoch-Myers from Weizmann’s Molecular Cell Biology Department; Dr Simon Gritsch, Dr Masashi Nomura, Hannah R. Weisman, Dr Nicolas Gonzalez Castro, Nicholas Druck, John J. Y. Lee, Ravindra Mylvaganam, Rachel Lee Servis, Jeremy Man Fung, Professor Christine K. Lee, Dr Hiroaki Nagashima, Professor Julie Miller, Dr Isabel Arrillaga-Romany, Dr David N. Louis and Professor Hiroaki Wakimoto from the Massachusetts General Hospital and Harvard Medical School in Boston, MA, USA; Dr Jerome Fortin from Princess Margaret Cancer Centre in Toronto and McGill University in Montreal, Canada; Dr Ramya Raviram, Professor Dan A. Landau and Dr Daniel P. Cahill from the New York Genome Center; Will Pisano and Professor Keith L. Ligon from Brigham and Women’s Hospital in Boston, MA, USA; Professor Patrick Y. Wen from the Dana-Farber Cancer Institute, Harvard Medical School, Boston; Professor Tak W. Mak from the University of Hong Kong; and Professor Marc Sanson and Dr Mehdi Touat from the Sorbonne University, Paris.

Dr Avishay Spitzer
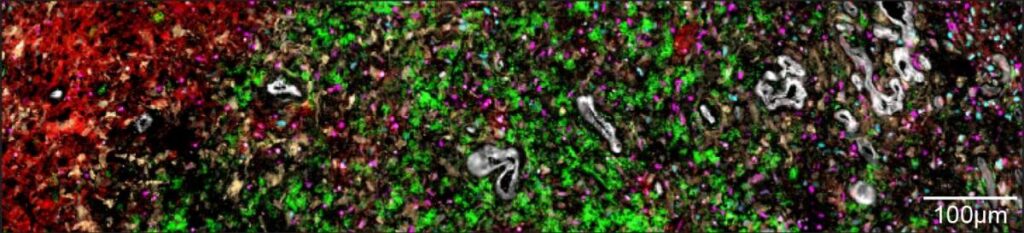
The layers of glioblastoma tumours: The inner layer contains the core of the tumour, made up of necrotic cells that receive no oxygen supply (red); the second layer contains cancerous cells that mimic those of the embryonic connective tissue (yellow); the third layer contains immune cells (green) and blood cells (white); cells in the tumour’s fourth layer receive oxygen and imitate healthy brain tissue (pink and blue)

(l-r) Dr Itay Tirosh, Dr Rouven Hoefflin, Dr Alissa Greenwald and Noam Galili Darnell






