March 20, 2021
A new method is set to reveal the hidden first stages of embryonic development – from a tiny ball of cells to organ growth – this unique research is from the Weizmann institute of Science and recently published in Nature.
To observe how a tiny ball of identical cells on its way to becoming a mammalian embryo first attaches to an awaiting uterine wall and develops into a nervous system, heart, stomach and limbs, is a highly-sought grail in the field of embryonic development for nearly 100 years.
Now Weizmann’s Professor Jacob Hanna and his group have accomplished this feat.
The method they created for growing mouse embryos outside the womb during the initial stages after embryo implantation will give researchers an unprecedented tool for understanding the development program encoded in the genes, and may provide detailed insight into birth and developmental defects as well as those involved in embryo implantation.
Hanna, who is in the Institute’s Molecular Genetics Department, explained that much of what is known about mammalian embryonic development today comes either from observing the process in non-mammals like frogs or fish that lay transparent eggs, or by obtaining static images from dissected mouse embryos and adding them together.
“The idea of growing early embryos outside the uterus has been around since before the 1930s, but experiments based on these proposals had limited success and the embryos tended to be abnormal,” he added.
Hanna’s team decided to renew that effort in order to advance the research in his lab, which focuses on the way the development program is enacted in embryonic stem cells. Over seven years, through trial and error, fine-tuning and double-checking, his team came up with a two-step process in which they were able to grow normally developing mouse embryos outside the uterus for six days – around a third of their 20-day gestation – by which time the embryos already had a well-defined body plan and visible organs.
“To us, that is the most mysterious and the most interesting part of embryonic development, and we can now observe it and experiment with it in amazing detail,” said Hanna.
The research was led by Alejandro Aguilera-Castrejon, Dr Bernardo Oldak, the late Dr Rada Massarwa and Dr Noa Novershtern in Hanna’s lab and Dr Itay Maza, a former student of Hanna’s now in the Rambam Health Care Campus of the Technion – Israel Institute of Technology.
For the first step, which lasted around two days, the researchers started with several-day old mouse embryos — right after they would have implanted in the uterus. At this stage the embryos were balls consisting of 250 identical stem cells.
These were placed on a special growth medium in a laboratory dish and the team got the balls to attach to this medium as they would to the uterine wall. With this step, they succeeded in duplicating the first stage of embryonic development, in which the embryo doubles and triples in size, as it differentiates into three layers: inner, middle and outer.
Beyond two days, as the embryos entered the next developmental stage – the formation of organs from each of the layers – they needed additional conditions.
For this second step, the scientists placed the embryos in a nutrient solution in tiny beakers, setting the beakers on rollers that kept the solutions in motion and continually mixed. That mixing seems to have helped keep the embryos, which were growing without maternal blood flow to the placenta, bathed in the nutrients. In addition to carefully regulating the nutrients in the beakers, the team learned in further experiments to closely control the gases, oxygen and carbon dioxide – not just the amounts, but the gas pressure as well.
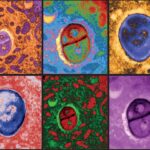
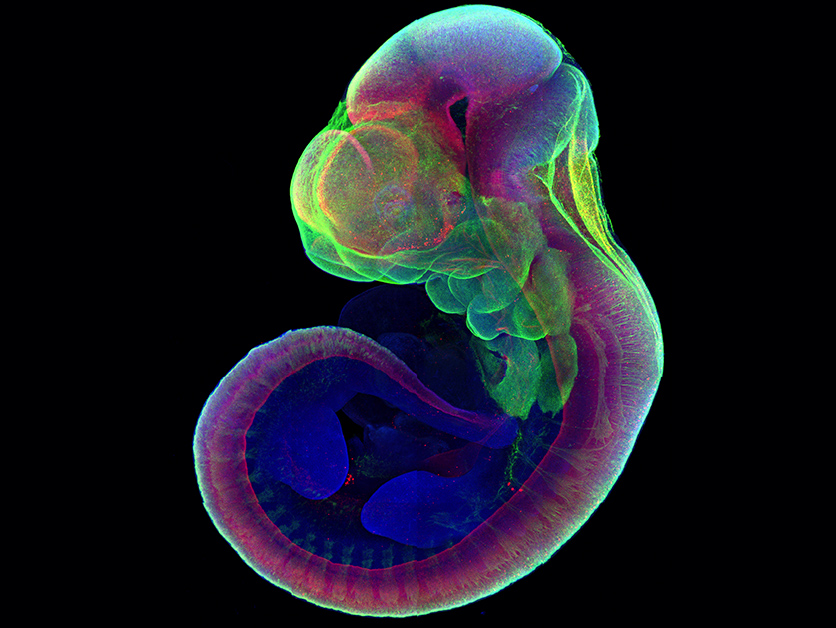
To check whether the developmental processes they were observing throughout the two steps were normal, the team conducted careful comparisons with embryos removed from pregnant mice in the relevant time period, showing that both the separation into layers and the organ formation were all but identical in the two groups. In subsequent experiments, they inserted into the embryos genes that labelled the growing organs in fluorescent colours.
The success of this attempt suggested that further experiments with this system involving various genetic and other manipulations should produce reliable results.
“We think you can inject genes or other elements into the cells, alter the conditions or infect the embryo with a virus, and the system we demonstrated will give you results consistent with development inside a mouse uterus,” said Hanna.
“If you give an embryo the right conditions, its genetic code will function like a pre-set line of dominos, arranged to fall one after the other,” he added.
“Our aim was to recreate those conditions, and now we can watch, in real time, as each domino hits the next one in line.
“Amongst other things, the method will lower the cost and speed up the process of research in the field of developmental biology, as well as reducing the need for lab animals,” Hanna said.
In fact, the next step in Hanna’s lab will be to see if they can skip the step of removing embryos from pregnant mice. He and his team intend to try to create artificial embryos made from stem cells for use in this research. They hope to put their new method to work to answer such questions as why so many pregnancies fail to implant, why the window for implantation is so short, how stem cells gradually lose their ‘stemness’ as differentiation progresses and what conditions in gestation may later lead to developmental disorders.
Also participating in this research were Tom Shani, Shadi Tarazi, Jonathan Bayerl, Valeriya Chugaeva, Dr Muneef Ayyash, Shahd Ashouokhi, Daoud Sheban, Nir Livnat, Dr Lior Lasman, Sergey Viukov and Dr Mirie Zerbib of the Weizmann Institute of Science’s Molecular Genetics Department; Dr Yonatan Stelzer, Dr Yoach Rais and Dr Saifeng Cheng of the Molecular Cell Biology Department; Dr Yoseph Addadi and Dr Hadas Keren-Shaul of the Life Sciences Core Facilities Department; Dr Nadir Ghanem and Chen Itzkovich of Rambam Medical Center; Dr Sharon Slomovichof the Technion – Israel Institute of Technology; and Raanan Shlomo of Arad Technologies.
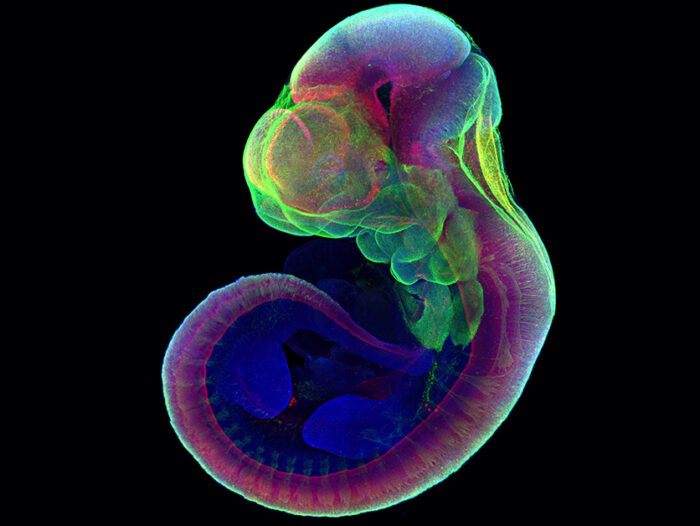
A mouse embryo grown six days outside the uterus is stained at the end point (day 11) for markers of development, revealing normal expression patterns of these markers