September 7, 2023
A research team headed by Professor Jacob Hanna at the Weizmann Institute of Science has created complete models of human embryos from stem cells cultured in the lab – and managed to grow them outside the womb up to day 14.
Reported today in Nature, these synthetic embryo models had all the structures and compartments characteristic of this stage, including the placenta, yolk sac, chorionic sac and other external tissues that ensure the models’ dynamic and adequate growth.
Created without an egg, sperm or womb, these synthetic embryo models could open new avenues of research into infertility, drug testing and growth of tissues for transplant – as well as help scientists peer into the dramatic first weeks of embryonic development.
The work developed because cellular aggregates derived from human stem cells in previous studies could not be considered genuinely accurate human embryo models because they lacked nearly all the defining hallmarks of a post-implantation embryo.
In particular, they failed to contain several cell types that are essential to the embryo’s development, such as those that form the placenta and the chorionic sac. They also did not have the structural organisation characteristic of the embryo and revealed no dynamic ability to progress to the next developmental stage.
Given their authentic complexity, the human embryo models obtained by Hanna’s group may provide an unprecedented opportunity to shed new light on the embryo’s mysterious beginnings.
Little is known about the early embryo because it is so difficult to study, for both ethical and technical reasons, yet its initial stages are crucial to its future development. During these stages, the clump of cells that implants itself in the womb on the seventh day of its existence becomes, within three to four weeks, a well-structured embryo that already contains all the body organs.
“The drama is in the first month, the remaining eight months of pregnancy are mainly lots of growth,” Hanna said.
“But that first month is still largely a black box. Our stem cell–derived human embryo model offers an ethical and accessible way of peering into this box. It closely mimics the development of a real human embryo, particularly the emergence of its exquisitely fine architecture.”
Letting the embryo model say “Go!”
Hanna’s team built on their previous experience in creating synthetic stem cell–based models of mouse embryos. As in that research, the scientists made no use of fertilized eggs or a womb. Rather, they started out with human cells known as pluripotent stem cells, which have the potential to differentiate into many, though not all, cell types. Some were derived from adult skin cells that had been reverted to ‘stemness’. Others were the progeny of human stem cell lines that had been cultured for years in the lab.
The researchers then used Hanna’s recently developed method to reprogram pluripotent stem cells so as to turn the clock further back: to revert these cells to an even earlier state – known as the naïve state – in which they are capable of becoming anything, that is, specialising into any type of cell. This stage corresponds to day seven of the natural human embryo, around the time it implants itself in the womb. Hanna’s team had in fact been the first to start describing methods to generate human naïve stem cells, back in 2013; they continued to improve these methods, which stand at the heart of the current project, over the years.
The scientists divided the cells into three groups. The cells intended to develop into the embryo were left as is. The cells in each of the other groups were treated only with chemicals, without any need for genetic modification, so as to turn on certain genes, which was intended to cause these cells to differentiate toward one of three tissue types needed to sustain the embryo: placenta, yolk sac or the extraembryonic mesoderm membrane that ultimately creates the chorionic sac.
Soon after being mixed together under optimised, specifically developed conditions, the cells formed clumps, about one percent of which self-organised into complete embryo-like structures.
“An embryo is self-driven by definition; we don’t need to tell it what to do – we must only unleash its internally encoded potential,” Hanna said.
“It’s critical to mix in the right kinds of cells at the beginning, which can only be derived from naïve stem cells that have no developmental restrictions. Once you do that, the embryo-like model itself says, ‘Go!’”
The stem cell–based embryo-like structures (termed SEMs) developed normally outside the womb for eight days, reaching a developmental stage equivalent to day 14 in human embryonic development. That’s the point at which natural embryos acquire the internal structures that enable them to proceed to the next stage: developing the progenitors of body organs.
Complete human embryo models match classic diagrams in terms of structure and cell identity
When the researchers compared the inner organization of their stem cell–derived embryo models with illustrations and microscopic anatomy sections in classical embryology atlases from the 1960s, they found an uncanny structural resemblance between the models and the natural human embryos at the corresponding stage. Every compartment and supporting structure was not only there, but in the right place, size and shape. Even the cells that make the hormone used in pregnancy testing were there and active: When the scientists applied secretions from these cells to a commercial pregnancy test, it came out positive.
This implied that their models faithfully emulated the process by which an early embryo gains all the structures it needs for beginning its transformation into a foetus.
“Many failures of pregnancy occur in the first few weeks, often before the woman even knows she’s pregnant,” Hanna said.
“That’s also when many birth defects originate, even though they tend to be discovered much later. Our models can be used to reveal the biochemical and mechanical signals that ensure proper development at this early stage, and the ways in which that development can go wrong.”
In fact, the study has already produced a finding that may open a new direction of research into early pregnancy failure. The researchers discovered that if the embryo is not enveloped by placenta-forming cells in the right manner at day 3 of the protocol (corresponding to day 10 in natural embryonic development), its internal structures, such as the yolk sac, fail to properly develop.
“An embryo is not static. It must have the right cells in the right organization, and it must be able to progress – it’s about being and becoming,” Hanna said.
“Our complete embryo models will help researchers address the most basic questions about what determines its proper growth.”
This ethical approach to unlocking the mysteries of the very first stages of embryonic development could open numerous research paths. It might help reveal the causes of many birth defects and types of infertility. It could also lead to new technologies for growing transplant tissues and organs. And it could offer a way around experiments that cannot be performed on live embryos – for example, determining the effects of exposure to drugs or other substances on foetal development.
Taking part in this study were Dr Bernardo Oldak, Emilie Wildschutz, Dr Vladyslav Bondarenko, Alejandro Aguilera-Castrejon, Shadi Tarazi, Mehmet-Yunus Comar, Shahd Ashouokhi, Dmitry Lokshtanov, Dr Francesco Roncato, Sergey Viukov, Eitan Ariel, Max Rose, Nir Livnat, Dr Tom Shani, Carine Joubran, Roni Cohen and Dr Noa Novershtern from Hanna’s lab in Weizmann’s Molecular Genetics Department; Dr Yoseph Addadi and Dr Merav Kedmi of Weizmann’s Life Sciences Core Facilities Department; Dr Hadas Keren-Shaul of the Nancy and Stephen Grand Israel National Center for Personalized Medicine; Dr Cheng Zhao, Professor Sophie Petropoulos and Professor Fredrik Lanner of the Karolinska Institutet; and Professor Vincent Pasque of the University of Leuven, Belgium.

(l-r) Dr Noa Novershtern, Dr Vladyslav Bondarenko, Professor Jacob Hanna, Dr Bernardo Oldak and Emilie Wildschutz
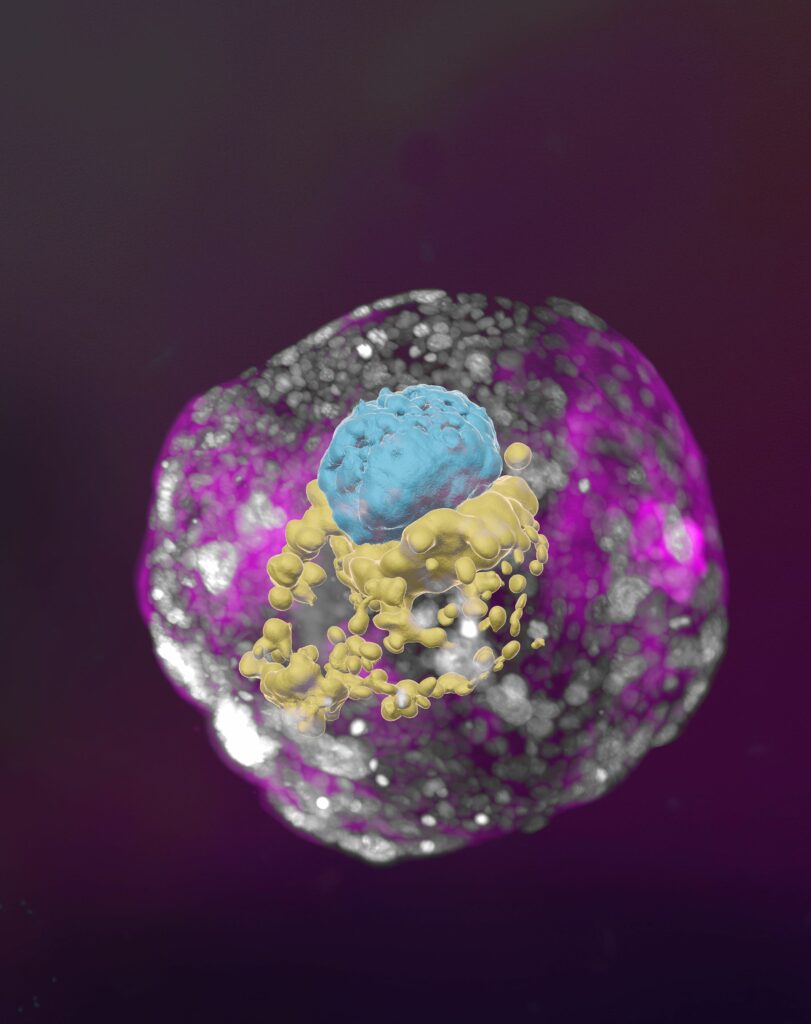
A stem cell–derived human embryo model at a developmental stage equivalent to that of a day 14 embryo. The model has all the compartments that define this stage: the yolk sac (yellow) and the part that will become the embryo itself, topped by the amnion (blue) – all enveloped by cells that will become the placenta (pink)
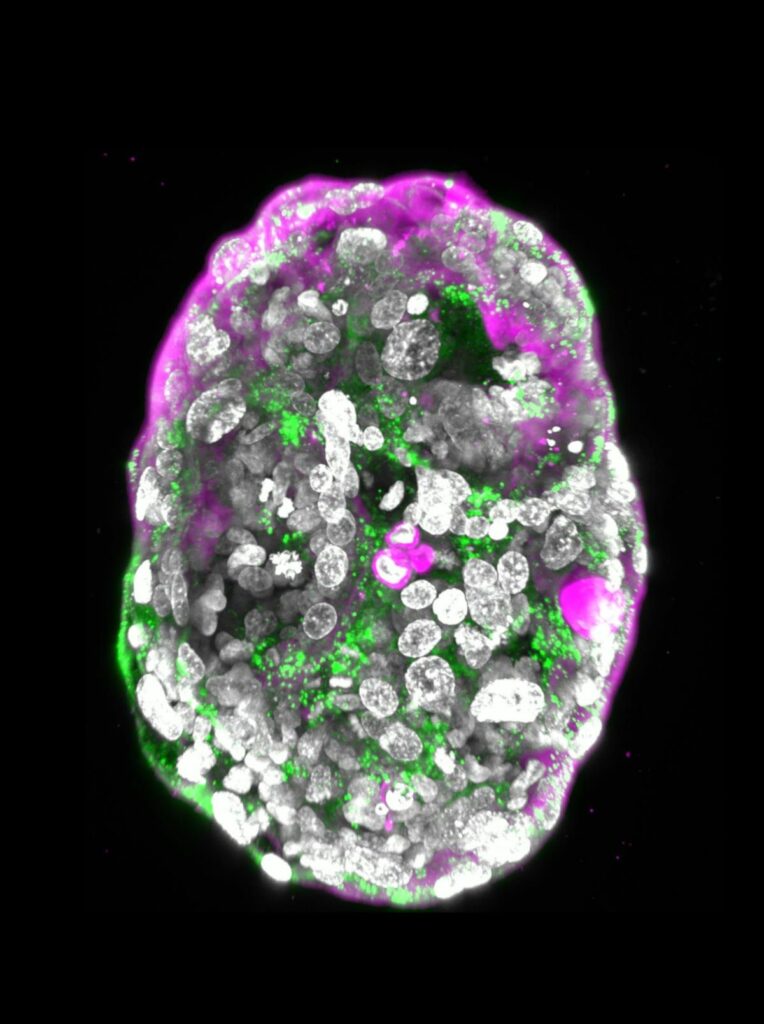
A human embryo model at a developmental stage equivalent to that of a human embryo at day 14, under the microscope. The image reveals the hormone used in pregnancy tests (green) and the outer layer slated to become the placenta (pink), which contains characteristic cavities, called lacunae. During normal pregnancy, the lacunae allow the exchange of nutrients and waste products between the maternal blood and the foetus
A stem cell–derived human embryo model at a developmental stage equivalent to that of a human embryo at day 12 has all the compartments typical of this stage. The top sphere contains the part that will become the embryo itself, capped by the amnion; the bottom sphere is the yolk sac; the interface between them is a critical structural feature of human embryonic development. The two spheres are encapsulated by a cellular layer that will become the placenta