Bacteria that cause life-threatening infections sometimes resort to the nastiest ploy of all: Stealing the human body’s defence weapons and exploiting them to their own advantage.
Researchers at the Weizmann Institute of Science have now uncovered one such strategy used by Salmonella, the findings recently published in Science.
When Salmonella bacteria penetrate the human gut, they can cause diarrhoea and other symptoms of food poisoning that often stay mild, but if they get into the bloodstream and from there into the liver, spleen and other body organs, they are liable to cause more severe disease that can be fatal.
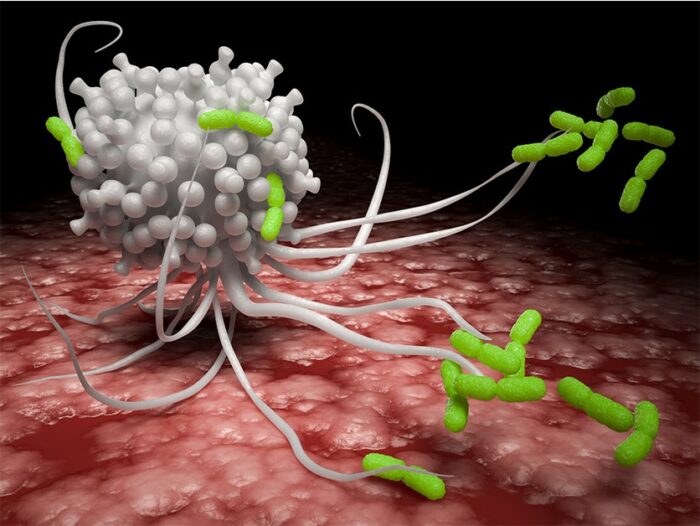
In the case of such invasion, large protective cells called macrophages try to stop the infection by swallowing the Salmonella whole. The bacteria, however, sometimes manage not only to survive but to thrive inside the macrophages, even converting them into incubators that facilitate their spread.
In a study led by doctoral student Gili Rosenberg in the lab of Dr Roi Avraham of Biological Regulation Department, the researchers started out by exposing macrophages to Salmonella and examining the changes that occur in these cells.
As the macrophages gear up to fight the bacteria, their metabolism undergoes such a major shift that they switch from producing energy in the cellular organelles called mitochondria to a massive burning of glucose. But when the scientists blocked this metabolic shift in the macrophages, they found – to their surprise – that the bacteria, instead of growing more aggressive, became less virulent.
This finding suggested that the virulence of Salmonella was somehow dependent on the metabolic shift. In other words, the very changes in cellular metabolism that were intended to help the macrophages deal with the infection could be hijacked and abused by Salmonella.
The scientists checked all the metabolites that accumulate in macrophages when they fight Salmonella, and they zeroed in on a compound called succinate. This compound is known to act as a signalling molecule that the macrophages use to activate their defences against invading bacteria: Succinate promotes the recruitment of the immune system and the generation of toxic inflammatory compounds that can kill the bacteria.
But as the scientists discovered, in the course of evolution the bacteria had learned to make use of this very molecule as a signal to become more virulent and to manipulate the contents of the macrophages to their own benefit. Succinate, as they found, activates certain bacterial genes, causing the Salmonella to grow a needle that punctures vacuoles –closed compartments within the macrophage that keep bacteria wrapped in ‘hazmat’ padding. The needle then secretes substances that neutralise the giant cell’s killing mechanism.
On top of this, succinate activates a mechanism that protects the Salmonella from antimicrobial peptides secreted within macrophages, so that the bacteria now feel free to treat the macrophage as a hotel, with all the amenities.
To confirm that these manipulations are indeed dependent on succinate, the scientists genetically engineered Salmonella to disable the transporter molecule that enables these bacteria to take up succinate, and compared the mutant bacteria to unaltered ones – that is, ones that can make full use of succinate. The mutant bacteria failed to survive inside macrophages and were much less effective at infecting mice than the unaltered ones.
In addition to providing insights into infection by Salmonella, the study’s results pave the way to investigating whether other intracellular bacteria hijack the immune metabolites that accumulate in macrophages following bacterial infection. These might include the bacteria responsible for tuberculosis, as well as Listeria, which can cause a form of meningitis and other severe infections, and Shigella, a common cause of children’s diarrhoea in Africa and South Asia.
The study’s findings may serve as a basis for developing antibacterial therapies to block the uptake of succinate by bacteria; such drugs would be more targeted than existing antibiotics.
“Whereas antibiotics kill all bacteria, including the good ones, a therapy based on blocking succinate can be aimed at killing only those that cause disease,” Rosenberg said.