
March 14, 2021
A collaborative research effort at the Weizmann Institute of Science has revealed that deadly malaria parasites’ are a pre-invasion strategy for softening up red blood cells.
Red blood cells are the body’s lifeline, but they also serve as the perfect hosts for one of the world’s deadliest organisms: the malaria parasite. About two weeks after infecting the body, Plasmodium falciparum launches its invasion, rapidly taking over the insides of masses of red blood cells. Then the disease turns life-threatening, especially for children: Around a thousand die of it every day.
Two research teams at the Weizmann Institute of Science have joined forces to reveal what enables the malaria parasite to mount such an effective takeover, with results recently published in Nature.
Dr Neta Regev-Rudzki and her team in the Biomolecular Sciences Department discovered that tiny sac-like ‘packages’ called extracellular vesicles are released by the Plasmodium falciparum parasite, and they contain a peculiar cargo: a cellular protein-degrading machine called a proteasome, which normally breaks down misfolded or unneeded proteins.
Departmental colleague Professor Michal Sharon, whose team specialises in studying proteasomes, suggested that the two labs combine their expertise to figure out what, if anything, those proteasomes are doing in the malaria packages.
The joint study – led by PhD student Elya Dekel from Regev-Rudzki’s lab and postdoctoral fellow Dr Dana Yaffe from Sharon’s lab – uncovered an extraordinary strategy by which the malaria parasite harnesses the proteasome for its own purposes, that is, priming red blood cells for the coming invasion.
These cells already possess unique properties that turn them into a particularly attractive host for Plasmodium falciparum. First of all, they lose most of their organelles by the time they mature, which means that they lack the means to defend themselves against the parasite. Second, these cells are able to squeeze through the smallest blood vessels to deliver oxygen to all body tissues, meaning they possess an enormous facility to undergo remodelling – a talent the parasite turns to its advantage.
After getting into the body through a mosquito bite, Plasmodium falciparum spends the first two weeks or so in the liver, where it advances into the next stage in its complex life cycle and multiplies. It then enters the bloodstream, invading a few mature red blood cells. From these the parasites send out an advance party – proteasomes encapsulated within the vesicles – to soften the membranes of neighbouring red blood cells, making them easier to penetrate. This preparatory step helps thousands of parasites to invade vast numbers of cells within seconds.
The researchers managed to expose the details of the pre-invasion. They established that malaria’s extracellular vesicles contain a version of the proteasome known as 20S; this version uses less energy and is smaller than others.
Working with Dr Irit Rosenhek-Goldian and Dr Sidney R. Cohen of the Chemical Research Support Department, the scientists developed a special visualisation technique based on atomic force microscopy, and they found that 20S pokes holes in the red blood cells’ cytoskeleton – the protein framework that holds the cellular membrane together – and dissolves some of its filaments.
The researchers identified four red blood cell proteins that get targeted for degradation by this proteasome. They also developed a machine-learning algorithm that distinguishes the cytoskeletons of red blood cells that had been altered by the secreted 20S, from those that had not.
Finally, to confirm that the softening of the red blood cells had indeed been produced by the proteasomes, the scientists blocked the active site of the 20S with an inhibitor prior to exposing the cells to the malarial extracellular vesicles. The inhibitor prevented the softening of the cellular membranes, limiting the invasion of the cells by the parasite and, consequently, the parasite’s growth.
This last result opens up a new direction in the search for malaria therapies: Preventing the parasite’s proteasomes from invading red blood cells.
The study’s findings may also prove relevant to other infectious diseases because subunits of the 20S proteasome have been found in extracellular vesicles released by different classes of parasites, for example, those causing sleeping sickness and leishmaniasis. Moreover, the results may be applicable to cancer, as the 20S has recently been detected in extracellular vesicles released by human cells that facilitate tumour growth.
The research team included Dr Gili Ben-Nissan, Dr Yifat Ofir-Birin, Dr Mattia Morandi, Yael Ohana-Daniel, Paula Abou Karam, Daniel Alfandari, Shimrit Malihi, Tal Block Temin, Dr Or-Yam Revach, Ariel Rudik and Dr Ori Avinoam of the Biomolecular Sciences Department; ; Dr Ron Rotkopf, Dr Ido Azuri and Dr Ziv Porat of the Life Sciences Core Facilities Department; Debakshi Mullick and Professor Nir S. Gov of the Chemical and Biological Physics Department; Dr Tamar Ziv of the Technion – Israel Institute of Technology; Dr Xavier Sisquella, Dr Matthew A. Pimentel, Dr Thomas Nebl and Dr Eugene Kapp of the University of Melbourne and Walter and Eliza Hall Institute of Medical Research; Giulia Bergamaschi and Professor Gijs Wuite of Vrije Universiteit Amsterdam; Dr Raya Sorkin of Tel Aviv University; and Dr Teresa Carvalho of La Trobe University, Melbourne.
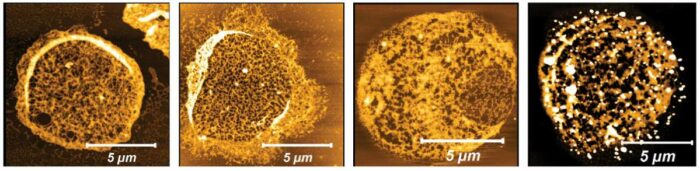
Cytoskeletons of red blood cells viewed with atomic force microscopy. Exposure to extracellular vesicles released by the malaria parasite caused filament melting (yellow, second from right) or the formation holes (black, far right), in contrast to controls (first and second from left)

(l-r) Dr Neta Regev-Rudzki and Professor Michal Sharon
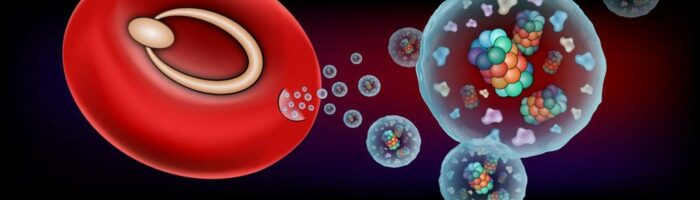
Schematic representation of a red blood cell infected by a ring-shaped malaria parasite that releases extracellular vesicles containing barrel-shaped 20S proteasomes





