July 1, 2016
Weizmann Institute of Science scientists have engineered bacteria that can create sugar from the greenhouse gas carbon dioxide (CO2).
Recently reported in Chem Europe the researchers posed the question: Is it possible to reprogram an organism that is found higher in the food chain, which consumes sugar and releases carbon dioxide, so it will consume carbon dioxide from the environment and produce the sugars it needs to build its body mass?
Dr Niv Antonovsky, who led the research from Professor Ron Milo’s lab at the Institute’s Plant and Environmental Sciences Department, said the ability to improve carbon fixation – where plants, algae and certain bacteria are able to pump carbon dioxide (CO2) from the environment, add solar or other energy and turn it into the sugars required as a starting point for life processes – is crucial for us to cope with future challenges, such as the need to supply food to a growing population on shrinking land resources while using less fossil fuel.
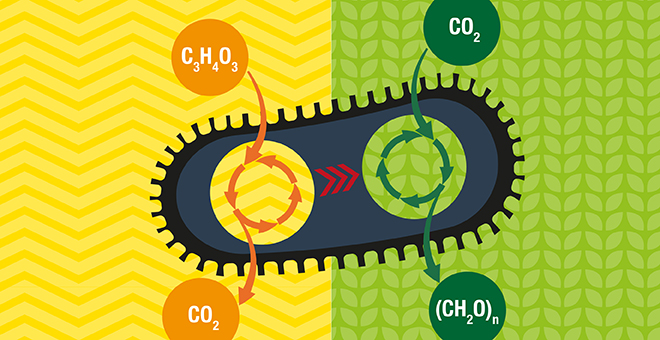
The Institute scientists rose to the challenge by inserting the metabolic pathway for carbon fixation and sugar production (called the Calvin cycle) into the bacterium E. coli, a known ‘consumer’ organism that eats sugar and releases carbon dioxide.
The metabolic pathway for carbon fixation is well known to scientists, and Milo and his group reckoned that with proper planning they would be able to attach the genes containing the information for building it into the bacterium’s genome. Yet the main enzyme used in plants to fix carbon, RuBisCO, utilises as a substrate for the CO2 fixation reaction, a metabolite which is toxic for the bacterial cells. Thus the design had to include precisely regulating the expression levels of the various genes across this multi-step pathway.
In one way the team’s well-thought-out plan was a resounding success: The bacteria did indeed produce the carbon fixation enzymes, and these were functional. But the machinery, as a whole, did not “deliver the goods”. Even though the carbon fixation machinery was expressed, the bacteria failed to use CO2 for sugar synthesis, relying instead on an external supply of sugar.
“Of course, we were dealing with an organism that has evolved over millions of years to eat sugar, not CO2, so we turned to evolution to help us create the system we intended,” said Antonovsky.
Antonovsky, Milo and the team, including Shmuel Gleizer, Arren Bar-Even, Yehudit Zohar, Elad Herz and others, next designed tanks called ‘chemostats’ in which they grew the bacteria, gradually nudging them into developing an appetite for CO2.
Initially, along with ample bubbles of CO2, the bacteria in the tanks were offered a large amount of pyruvate, which is an energy source, as well as barely enough sugar to survive. By changing the conditions of their environment and stressing them, the scientists forced the bacteria to learn, by adaptation and development, to use the more abundant material in their environment.
A month went by, and things remained fairly static. The bacteria seemed to not “get the hint”. But at around a month and a half, some bacteria showed signs of doing more than “just surviving”. By the third month the scientists were able to wean the evolved bacteria from the sugar and raise them on CO2 and pyruvate alone. Isotope labeling of the carbon dioxide molecules revealed that the bacteria were indeed using CO2 to create a significant portion of their body mass, including all the sugars needed to make the cell.
Milo explained that when the scientists sequenced the genomes of the evolved bacteria, they found many changes scattered throughout the bacterial chromosomes.
“They were completely different from what we had predicted. It took us two years of hard work to understand which of these are essential and to unravel the ‘logic’ involved in their evolution,” said Milo.
Repeating the experiment (and again waiting months) gave the scientists essential clues for identifying the mutations necessary for changing the E. coli diet from one based on sugar to one using carbon dioxide.
“The ability to program or reengineer E. coli to fix carbon could give researchers a new toolbox for studying and improving this basic process.” Although currently the bacteria release CO2back into the atmosphere, the team envisions that in the future their insights might be applied to creating microorganisms that soak up atmospheric CO2and convert it into stored energy or to achieving crops with carbon fixing pathways, resulting in higher yields and better adaption to feeding humanity,” Milo concluded.

Professor Ron Milo, Weizmann Institute of Science